How to Find Experimental Molar Mass
Calculate its theoretical molar mass based on this formula. Calculate the experimental molar mass of butane by dividing the mass M by n the number of moles.

1 3 Calculating Molar Mass Of A Gas Using Pv Nrt Youtube
Calculate diatomic elements Remember that seven elements hydrogen nitrogen oxygen fluorine chlorine.

. Predicted theoretical molar H2 volume. The molar mass of a molecule can be determined using atomic masses. Determine the moles of unknown the solute from the molarityof the solution and the volume in liters of the solution.
Recorded as 7492 mm of Hg. Make a data table in either Word or Excel containing the information below. Well since you got a mass of 243040 g for magnesium and 000538 mols you can then compare it to the molar mass of magnesium.
Then use the molality equation to calculate the moles of solute. To calculate your experimental molar volume of H 2 under STP conditions your value for V 2 is the value you will divide by the number of moles of H 2 generated. Calculation of the molar volume volume of one mole of H2 gas at STP conditions temperature of 0 C 273 K and pressure of 1 atm 760 torr will also be done.
Students will Learn how to use the ideal gas law and Daltons law of partial pressures to calculate the molar mass of a gas Practice collection of a gas using water displacement. For the data. Find molar mass of an element Find the molar mass of an element.
Calculate the molar volume of the gas at STP. ___6583_____ gmol MM 0079 g 00012 mol 6583 gmol 14. 15 20 300 milligrams of Mass.
The molecular weight of this compound is 18018gmol. MgCl 2aq H 2g Moles of H 2g Moles of Mg 1 mole H 2 1 mole Mg 000154 moles Temperature of H 2g 22C 295 K T 1 Calculate. List the known quantities and plan the problem.
Find molar mass Step 1. Calculation of Percent Error. Molar mass of an element Relative atomic mass x.
List the known quantities and plan the problem. Therefore 6219 x 10-3 mol of acid were present before the titration. Molar mass of an element is given as below.
Click to see full answer. This can be done simply by the addition of molar masses of each atom present. This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles.
Gmol Use the freeing point depression to calculate the molality of the solution. On an experimental bases we have found that 001397 moles of magnesium has combined with 001419 moles of. Enter Value in Concentration textbox and Select the Unit of Concentration Enter Value in Volume textbox and Select the Unit of Volume to Calculate the Mass.
Calculate the number of moles of butane collected using RT PV n 2. This gives a ratio in which no number is less than 1. This can be done simply by the addition of molar masses of each atom present.
In grams molar mass is numerically equal to. Also to know is how do you find the molar mass of an. Finding the Mass from Concentration Volume.
This relationship can be given as follows. What is the molar mass of the acid. Determine the molar concentration of the unknown in the solutionfrom the observed osmotic pressure.
The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula. Using the mass calculated in Calculation 7 and the number of moles calculated in Calculation 12 calculate the experimental molar mass of butane. The moles of base titrant can be determined from the molarity of the base solution multiplied by the volume of titrant required to reach the equivalence point or moles base M.
Calculate the molar ratios To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. 243040 g 000538 mols 4517 gmol. Known mass H 2 O 218 g 0218 kg mass solute 387 g Unknown molar mass solute.
Mass of flask foil cover condensed ethanol 8 15 В 10069731004973 1102 113 147 1128 12 290mL 290 m2. Mass of flaskfoil cover e B. Divide the molar mass for the molecular formula by the empirical formula mass.
Mass volume concentration exp. Molar Mass and Vapor Density Data Trial 1 Trial 2 P Barometric pressure atm A. The number of moles of magnesium reacted is based on the mass of magnesium taken and the molar mass of magnesium 2431 g per mole of Mg.
To calculate the molality of the solution. Consider chemical compounds without. 224 L mol _Error experimental theoretical theoretical 100 _Error experimental_Molar_Volume_H 2.
Empirical formula weight 1 x 1201gmol 2 x 101gmol 1 x 1600gmol 3002gmol. Then use the molality equation to calculate the moles of solute. Calculate the accepted value for the molar mass of butane using a periodic table.
In this experiment the molar mass of butane is determined using the ideal gas law and Daltons law of partial pressures. Determine the molar mass from the mass of the unknown and thenumber of moles of unknown. Butane has the molecular formula C 4 H 10.
Calculate the molar mass of the solute. Then divide the grams of solute by the moles to determine the molar mass. Molar mass of an element is given as below.
Mass of magnesium metal 00369 grams Atomic mass of magnesium metal 24 gramsmol Moles of magnesium metal 000154 moles Mg s 2HCl aq. You will calculate the ideal gas constant R using the ideal gas equation and the experimental values of pressure volume temperature and number of moles of H2 gas. Reaction is known the molar mass of the unknown acid can be calculated by modification of equation 3.
How do you calculate experimental molar mass. When the Mass is unknown you can use this section. Relative atomic mass is the mass of an atom relative to the mass of Carbon-12 atom and it has no units.
Mol Acid mol Base 0006219mol. The molar mass of a molecule can be determined using atomic masses.

Chemistry 101 Determining Molecular Formula From Experimental Data Youtube

1 3 Determine The Molar Mass Of A Gas Experimentally Youtube
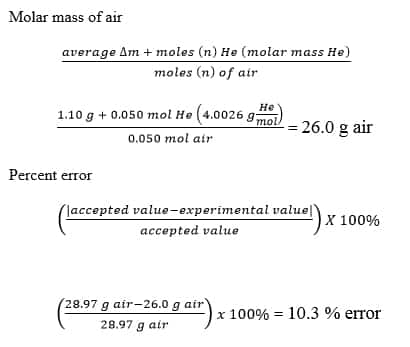
No comments for "How to Find Experimental Molar Mass"
Post a Comment